


...the spread of antimicrobial resistant bacteria there needs to be a significant decrease in antibiotic use in wound care.
While the World Health Organisation is addressing AMR with a Global Action Plan, there is a lot of room to contribute to the prevention of AMR in the wound care sector 1 To be truly effective against AMR, action must be taken at every level of wound care, from wound specialists to wound nurses.
The European Wound Management Association recommends avoiding the unnecessary usage of antibiotics through adequate infection prevention/management and appropriate hygiene protocols.2
With the right tools for infection prevention and management in wound care, the unnecessary use of antibiotics may be avoidable. Through its brands, Cutimed® and Leukomed®, Essity offers a comprehensive range of wound care products that effectively prevent and manage infection with no known risk of further contributing to antimicrobial resistance.
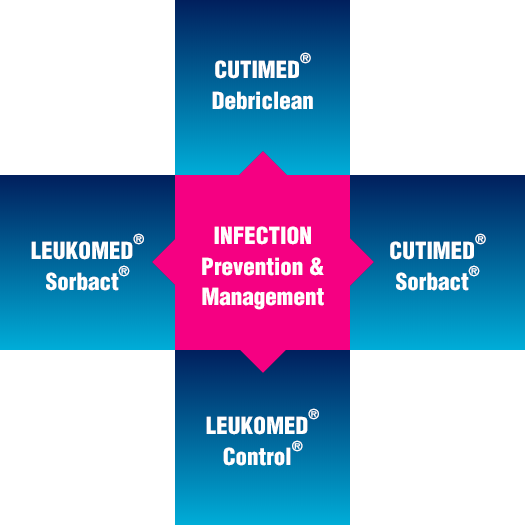
Cutimed® and Leukomed® offer an extensive range of effective products in wound management and infection control which may help avoid excessive use of antibiotics in wound care.

Surgical post-operative dresssing for the reduction of bacterial colonisation with a purely physical mode of action

Infection management for chronic wounds, with a purely physical mode of action

Transparent wound dressing for effective infection risk control.

Worlds first spools and snap rings that actively combat bacteria

Mechanical debridement pad.
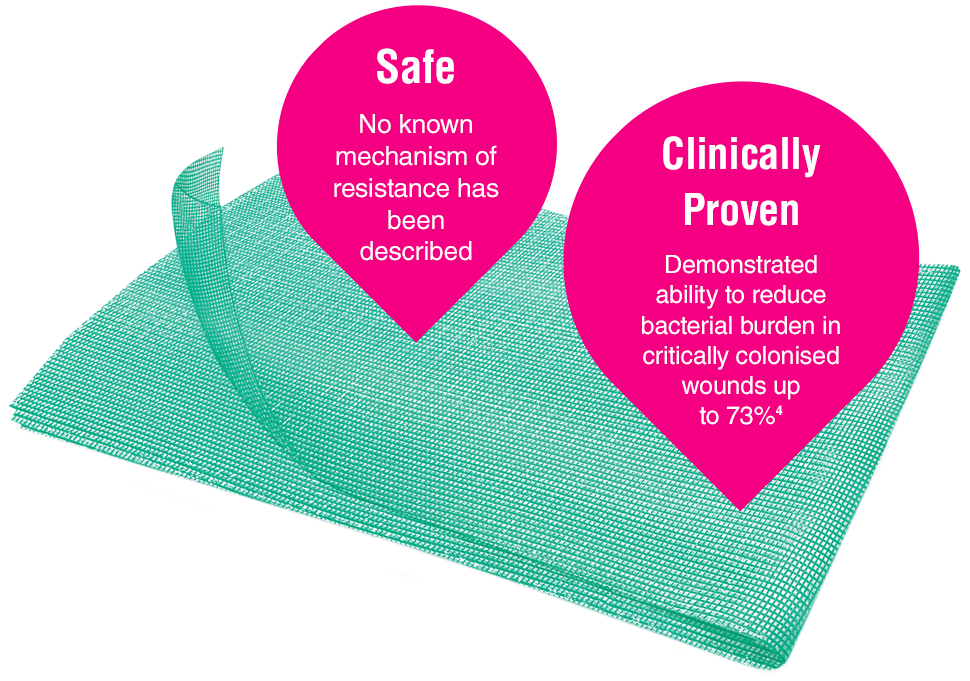
Leukomed® Sorbact® and Cutimed® Sorbact® utilise the safe and effective Sorbact® technology that binds bacteria with a purely physical mode of action. Sorbact® Technology removes bacteria without releasing possibly harmful endotoxins 3
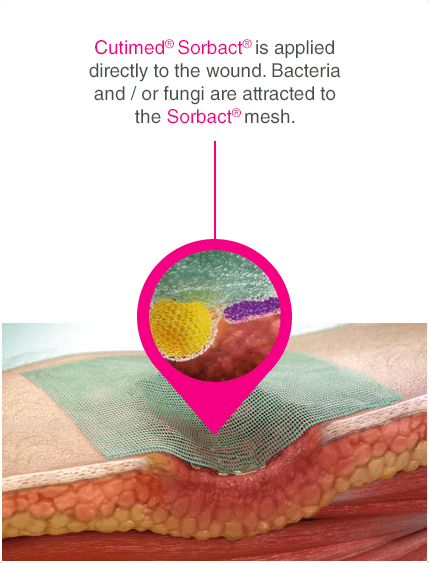
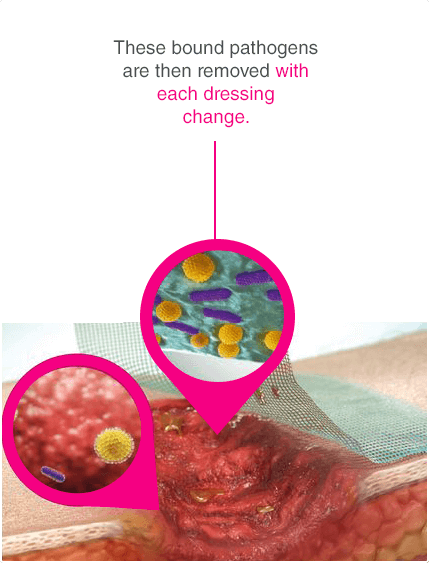
Advanced chronic wound dressing for safe and effective wound management with a purely physical mode of action

Innovative surgical post-operative dressing for the reduction of bacterial colonisation with a purely physical mode of action.
Indications
All post/operative and traumatic wounds with dry to low exudate levels:

Essity (T/A BSN medical Aust. Pty Ltd)
PO Box 337
Mount Waverley, Victoria, 3149
Australia
Australia:
customerservice.AU@essity.com
T: 1300 276 633 F: 1300 998 830
New Zealand:
customerservice.NZ@essity.com
T: 0508 276 111 F: 0508 998 830